skin replacement surgery
Improved Quality of Life
is the Promise
Standard of care in burn and reconstructive surgery may be scarce, lead to severe scarring and is costly over time. After more than 20 years of research and development, CUTISS promises to take skin surgery to the next level and revolutionize current treatments with our lead product denovoSkin™.
denovoSkin™ is a personalized, bio-engineered skin graft. Classified as an advanced therapy medicinal product (ATMP) it can be bio-engineered in large quantities, starting from a small, stamp-sized piece of the patient’s healthy skin. denovoSkin™ is composed of an epidermis (top, protective layer = life) and a dermis (the structural below layer = quality of life) for the permanent treatment of skin defects and injuries.
It promises to grow with the patient, limit scarring, and drastically reduce the number of follow up corrective surgeries required, particularly in children. The outcome is potentially life-saving and life-changing.
denovoSkin™ is in late-stage clinical trials in Switzerland and the European Union and also accessible for compassionate use. denovoSkin™ has been granted Orphan Drug Designation for the treatment of burns from Swissmedic, EMA, and FDA.

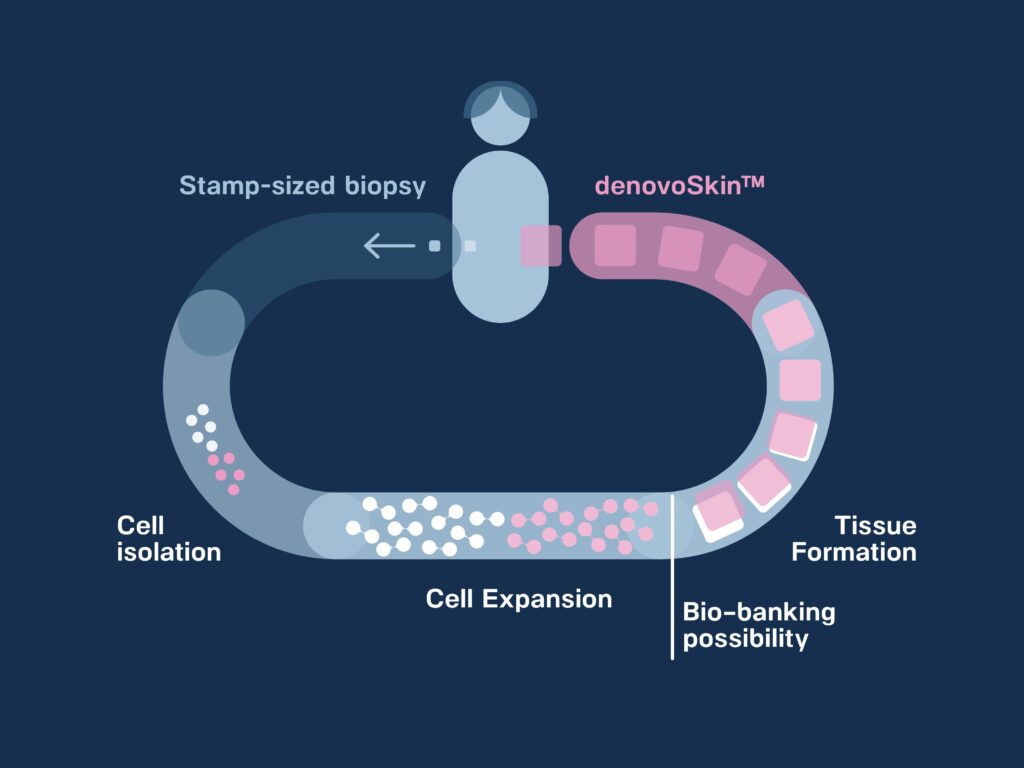
Proprietary manufacturing process
To bio-engineer denovoSkin™, a small biopsy of healthy skin is harvested from the patient. The biopsy is processed to isolate epidermal and dermal cells. The cells are expanded in vitro (can be bio-banked for further use) and thereafter used in combination with a hydrogel for tissue formation.
denovoSkin™, a dermo-epidermal personalizes skin graft, is now ready for grafting. Its mode of action is permanent take.
Automated manufacturing
for scale up
Skin is our largest organ – adults carry around 3.6 kilograms and 2 square meters of it. The bio-engineering of personalized skin tissue therapy faces scale up challenges.
At CUTISS we are working on the translation of the current manual manufacturing process into an automated, fully closed one.
Automation is expected to decrease production costs and time, ensure robustness of the process, and allow for the de-centralization of manufacturing.
We have developed our own unique approach to bioengineering human skin tissue composed of three modules: cell isolation (Semotiss™, CUTISS proprietary technology), cell expansion (Quantum®, TERUMO), and skin tissue formation (denovoCast™, CUTISS proprietary technology).


Skin pigmentation restoration
Acquired under a global, exclusive commercialization license from IBSA PHARMA in 2022 – Viticell® is CE marked for the cell therapy-based treatment of vitiligo and dyspigmented burn scars at point-of-care.

