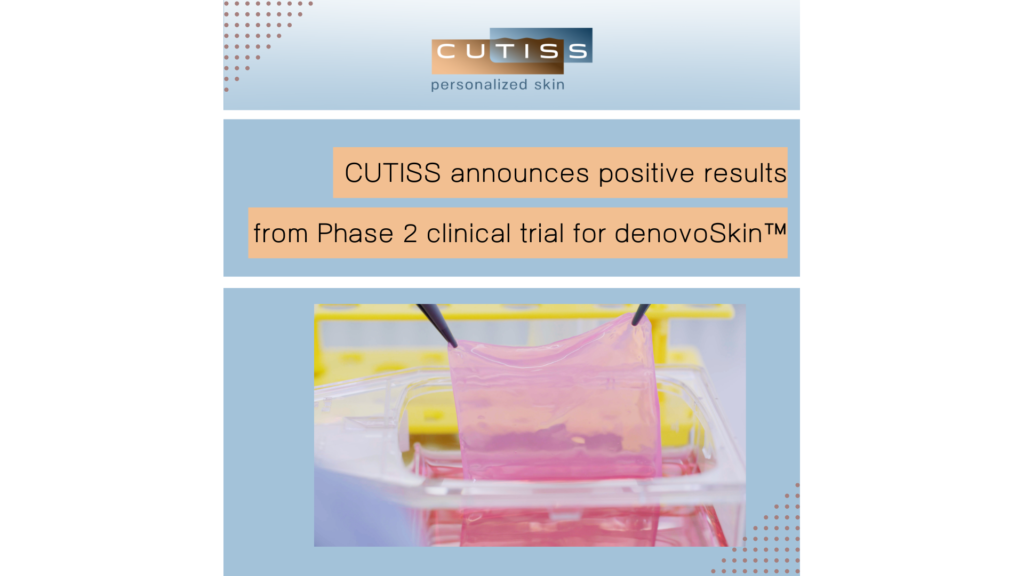
Positive one-year follow-up data from Phase 2 trial of denovoSkin™
Switzerland, 14 February 2024 – CUTISS AG, a Swiss clinical-stage life science company focused on skin regenerative medicine and tissue engineering, has announced positive one-year follow-up data from its Phase 2 clinical trial of the lead product denovoSkin™, in adult and adolescent severe burn patients. Following the announced positive primary endpoint in Q1 2023, the one-year readout […]
Positive one-year follow-up data from Phase 2 trial of denovoSkin™ Read More »