Skin surgery for cutaneous injuries and defects
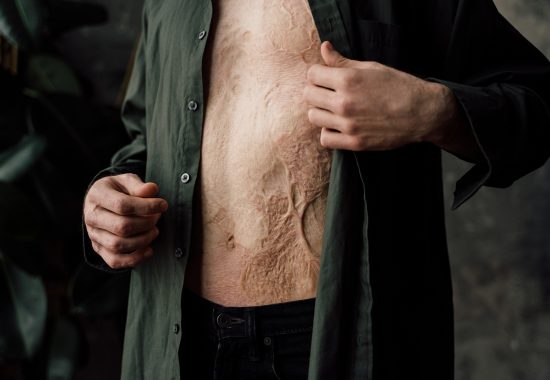
Skin is our body’s largest organ. Worldwide, millions of people suffer from large, deep skin defects requiring surgical interventions to restore skin function. Skin defects can be acute (burns and trauma) or elective (scar revisions, giant nevi or tumor resections, plastic surgery, etc.).
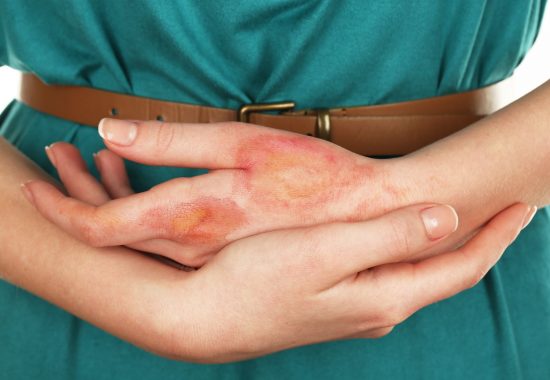
Burns are a global public health problem and major cause of death and disability globally, and number of plastic surgeries and skin cancers is growing globally.
The current standard of care in burn, plastic and reconstructive surgery – autografting – is often not available in sufficient quantities due to donor site shortage and yields skin grafts that are too thin and too often result in permanent painful, debilitating, disfiguring scars that frequently require follow up corrective surgeries, psychosocial rehabilitation and intense home care.
Clinical development program
denovoSkin™ is being clinically tested on burns and reconstructive indication in children and adults.
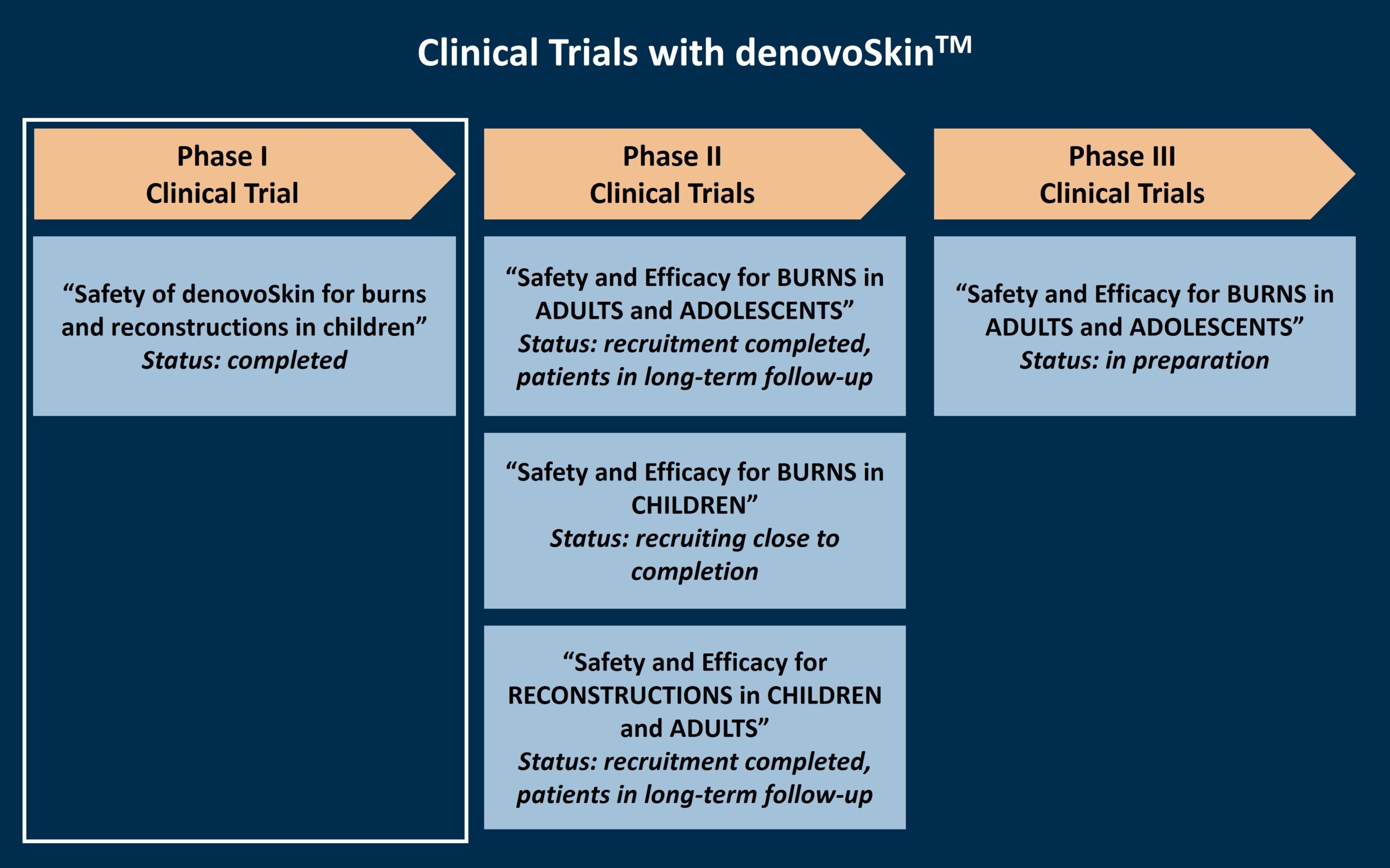
Phase I Clinical Trial – Safety Study
A phase I clinical trial for denovoSkin™ was completed with a five-year follow-up period in 2021, with 10 paediatric patients at the University Children’s Hospital Zurich, Switzerland. This study assessed the safety of the product in children and adults who had to undergo skin transplantation due to acute burns or reconstructive surgeries. An area of the wounds of the patient were treated with a maximum of one denovoSkin™ graft. The study was successfully completed, confirming the product’s safety in this first-in-man clinical trial.

Phase II Clinical Trials – Safety and Efficacy Studies
Following the successful completion of the phase I clinical trial, denovoSkin™ is further evaluated for its safety and efficacy in three parallel phase II clinical trials. These trials are very similar in their design, but differ in the patient population and indication. In all three phase II clinical trials, denovoSkin™ is compared to today’s standard of care, the transplantation of autologous split-thickness skin. For this, two comparable wound areas are selected, and one of the areas is treated with a maximum of one denovoSkin™ graft, and the other area with the standard of care. During the study visits, the two areas are analyzed and compared, for a total of 3 years.
 Phase III Clinical Trial – Safety and Efficacy Study
Phase III Clinical Trial – Safety and Efficacy StudyFollowing the anticipated successful completion of the phase II clinical trial for denovoSkin™ for burns in adults, a phase III clinical trial will be performed to further confirm the product’s safety and efficacy. This clinical trial is currently in preparation.
Named-patient basis treatment
Under special circumstances, there is a treatment option that allows the use of an unauthorised medicine, such as denovoSkin™ at its current development stage. Under strict conditions, denovoSkin™ can be made available directly from CUTISS to a physician treating a patient who suffers from a severe condition with no satisfactory authorised therapies and who cannot enter clinical trials. This is done on an individual basis under the direct responsibility of the doctor.
If you are a healthcare professional requesting more information on a potential named-patient treatment, please contact CUTISS.
Clinical and scientific publications
November 2023
Nature Reviews Disease Primers - Abstract

